The Fresh Test® is a diagnostic glucose beverage intended for gestational diabetes screening. Glucose tolerance tests are typically performed at 24-28 weeks gestation.
The Fresh Test® is a diagnostic glucose beverage intended for gestational diabetes screening. Glucose tolerance tests are typically performed at 24-28 weeks gestation.


The Fresh Test® is a patented glucose beverage formulated with high quality organic ingredients. We believe this is what makes the product well tolerated.
The Fresh Test® is a Class II Diagnostic product intended to screen and detect Diabetes Mellitus and Gestational Diabetes Mellitus. Patients are to follow an office's existing OGTT, oral glucose tolerance test protocol. The Fresh Test™ has proven exact equivalence to predicate diagnostic glucose beverages. Certificates of Analysis are available for every product lot.
Instructions For Use are referenced here and on product packaging. Fresh Test, LLC most frequently references the American Diabetes Associations guidelines and LabCorp's ACOG recommendations.

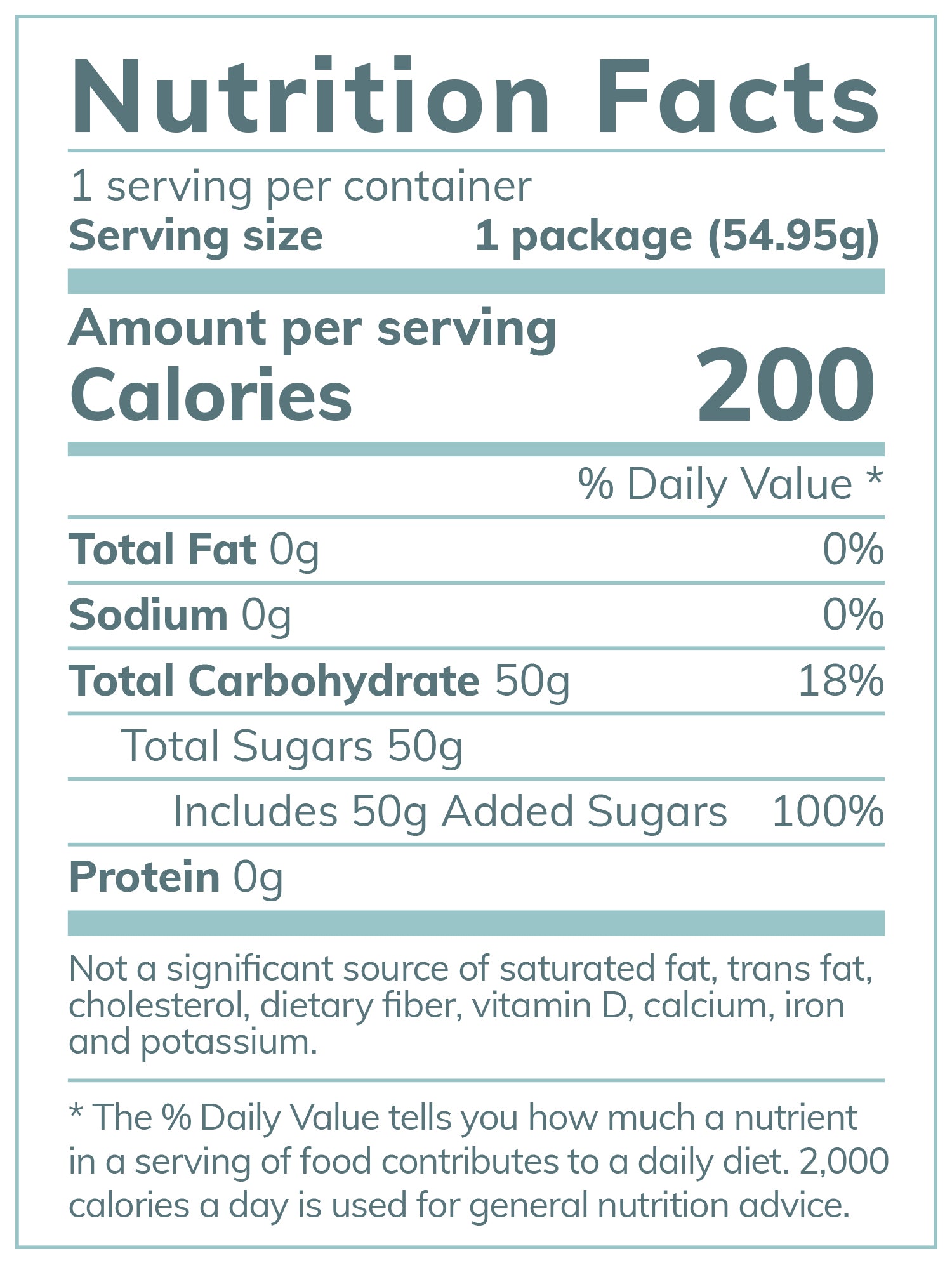
The Fresh Test’s ingredients are KOSHER, non-gmo, well-sourced and additive-free.
Our patent pending ingredient list includes Organic Non-GMO Glucose (derived from either corn or tapioca), Crystallized Lemon (citric acid, lemon oil, lemon juice) and Organic Peppermint Leaf. *If your patient has a corn or tapioca allergy, please notify our company to ensure they receive the correct product.
The Fresh Test's high quality ingredients have been known to make the Glucose Tolerance Test more enjoyable while easing nausea. In a manufacturer’s survey 356 out of 366 pregnant women enjoyed the taste; greatly improving the patient experience. Most importantly, The Fresh Test® is additive free, dye free and artificial flavoring free. There are no sodium benzoates, BVO, BPA or other unnecessary preservatives.

Fresh Test, LLC goes to great lengths to ensure exact 50-gram, 75-gram and 100-gram glucose products. In addition to FDA Approved manufacturers, Fresh Test LLC also uses third-party ISO 17025 laboratories to certify the glucose content of each batch of product. Fresh Test’s Medical Device and Pharmaceutical manufacturing facilities have been thoroughly vetted by their regulatory board. If you’re a provider or laboratory, please contact info@thefreshtest.com for more information on our product, a sample and Certificates of Analysis.

Women are becoming increasingly health-conscious and choosing to consume healthy and organic products, especially during pregnancy. The average pregnant woman knows to avoid caffeine, alcohol, toxic cleaning agents and when possible to avoid processed foods. This gives reason for us to pause and reconsider glucose beverages that contain antiquated or unnecessary additives used to lengthen shelf life and prevent ingredient separation. Many glucose beverages were formulated in the 1970s and have had few changes since. Being that glucose tolerance testing is extremely important in pregnancy, yet women are requesting a more palatable beverage, Fresh Test LLC felt it was critical to develop a clean and delicious product with The Fresh Test™. Please note, food alternatives such as juice, jelly beans or Twizzlers have not been validated for use in the oral glucose tolerance test. These products do not contain exact amounts of glucose but may contain fructose and sucrose (disaccharide) which have varying plasma glucose responses. Please reach out for more information. In the past, here are some of the ingredients you may find in traditional glucose (glucola) beverages
Dextrose derived from genetically modified Bt-Corn - GMO Dextrose is derived from conventional (GMO) corn, known as Bacillus thuringiensis (Bt) corn. Bt-corn is genetically modified with Bt to help corn plants synthesize their bacterial protein to kill pests. Bt-corn is categorized as a pesticide by the EPA.
Brominated Vegetable Oil (BVO) - BVO contains bromine, which is patented as a flame retardant. Bromine or its metabolites has the potential to bioaccumulate in the body and is toxic to primary hepatocytes and the endocrine system. It was designated by the FDA as Generally Recognized as Safe (GRAS) in 1958. The GRAS designation for BVO was withdrawn in 1970 due to poisonous amounts in animal feed. BVO is banned as a food additive in Europe and Japan. Polybrominated diphenyl ether flame retardants can have negative health effects, including infertility, miscarriage, preterm birth, and neurodevelopmental impairment.15
Red Dye #40 and Yellow Dye #6 - Food dyes have been associated with attention deficit/hyperactivity symptoms in children, migraines, and allergic responses. They may contain benzidine, a human and animal carcinogen. Studies in rats showed that food dyes activate the immune system and accelerate the production of adrenal and testicular tumors.
Butylated Hydroxyanisole (BHA) - BHA is considered a potential human carcinogen based on evidence in experimental animals. BHA was listed in 1990 as a chemical known to the State of California to cause cancer under the Safe Drinking Water and Toxic Enforcement Act of 1986.
Sodium Benzoate - Sodium benzoate is a commonly used preservative that is considered safe by the FDA when used within approved limits. However, some consumers prefer to avoid preservatives when possible. The Fresh Test is made without sodium benzoate or other added preservatives, offering a simple, dye-free alternative for those seeking a more ingredient-conscious option.
Sodium Hexametaphosphate (SHMP) - SHMP, a polyphosphate, is found in foods as a sequestrant, thickener, and emulsifier. It's also typically used in cosmetics and oral care as a corrosion inhibitor. In acute studies, rabbits fed 10% SHMP had pale and swollen kidneys.
BPA found in plastic packaging - BPA is a chemical with reported adverse associations such as infertility, miscarriage, and neurodevelopmental impairment.